hermes studie | ziltivekimab vs Hermes hermes studie The HERMES trial is an international, multicentre, parallel group, randomized, double-blind, study in patients with HFpEF and HFmrEF, evaluating the effect of ziltivekimab 15mg compared with placebo, given once a month, in addition to . $5,799.00
0 · ziltivekimab vs Hermes
1 · Hermes trial ziltivekimab
2 · Hermes heart failure
3 · Hermes consortium
4 · Hermes cardiology
$12K+
ziltivekimab vs Hermes
The HERMES trial is an international, multicentre, parallel group, randomized, double-blind, study in patients with HFpEF and HFmrEF, evaluating the effect of ziltivekimab 15mg compared with placebo, given once a month, in addition to .
Hermes trial ziltivekimab
HERMES is a single-centre phase II trial of magnetic resonance-guided SBRT in men with localised prostate cancer . Forty-six men will be treated on the MR-linac and will be .HERMES is an international collaboration to investigate the genetic basis of heart failure. This unique global effort currently comprises 57 population-based cohorts, case-control studies and randomized clinical trials, including over 140,000 .
Recent randomised trials have demonstrated that anti-cytokine and anti-inflammatory therapies can reduce cardiovascular event rates, and . Our analysis of data from the HERMES collaboration suggests that EVT is a safe and effective treatment option for patients with acute ischemic stroke with moderate‐to‐severe deficits due to an intracranial isolated ICA‐I occlusion, that is, ICA‐occlusion without involvement of the middle or anterior cerebral artery. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021 May 29;397 .
hermes (nct05636176) Effects of ziltivekimab versus placebo on morbidity and mortality in patients with heart failure with mildly reduced or preserved ejection fraction and systemic inflammation (HERMES).
Die Studie beschreibt die angestrebte Lösung, in dem sie die groben Ziele definiert, möglichen Lösungsvarianten aufführt und diese dann bewertet. Sie bildet die Grundlage für die . In sum, based on the recent RESCUE data, the opportunity has arisen to move past CANTOS and IL-1β inhibition to address in ZEUS whether targeting IL-6 can provide even .
Hermes heart failure
Hermes consortium
michael kors nylon tote
The HERMES trial is an international, multicentre, parallel group, randomized, double-blind, study in patients with HFpEF and HFmrEF, evaluating the effect of ziltivekimab 15mg compared with placebo, given once a month, in addition to standard care, on the primary composite outcome of time to first occurrence of cardiovascular death, heart .
HERMES is a single-centre phase II trial of magnetic resonance-guided SBRT in men with localised prostate cancer . Forty-six men will be treated on the MR-linac and will be randomised between 36.25 Gy in five fractions over 10 days, with clinical target volume target of 40 Gy, and 24 Gy in two fractions over 8 days.
HERMES is an international collaboration to investigate the genetic basis of heart failure. This unique global effort currently comprises 57 population-based cohorts, case-control studies and randomized clinical trials, including over 140,000 heart failure cases. Recent randomised trials have demonstrated that anti-cytokine and anti-inflammatory therapies can reduce cardiovascular event rates, and evolving genetic, murine, translational, and human epidemiological data suggest that IL-6 .
IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021 May 29;397 (10289):2060-2069. doi: 10.1016/S0140-6736 (21)00520-1. Epub 2021 May 17.Die Studie beschreibt die angestrebte Lösung, in dem sie die groben Ziele definiert, möglichen Lösungsvarianten aufführt und diese dann bewertet. Sie bildet die Grundlage für die Entscheidung, ob ein Projekt freigegeben wird oder nicht.
hermes (nct05636176) Effects of ziltivekimab versus placebo on morbidity and mortality in patients with heart failure with mildly reduced or preserved ejection fraction and systemic inflammation (HERMES). In sum, based on the recent RESCUE data, the opportunity has arisen to move past CANTOS and IL-1β inhibition to address in ZEUS whether targeting IL-6 can provide even larger reductions in cardiovascular event rates.Background: We compared the tolerability and efficacy of erenumab, a monoclonal antibody binding to the calcitonin gene-related peptide receptor, to topiramate for migraine prophylaxis in adults. Methods: HER-MES was a 24-week, randomised, double-blind, double-dummy, controlled trial conducted in 82 sites in Germany.
HERMES is a global collaboration aiming to (i) identify the genetic determinants of heart failure; (ii) generate insights into the causal pathways leading to heart failure and enable genetic approaches to target prioritization; and (iii) develop genomic .The HERMES trial is an international, multicentre, parallel group, randomized, double-blind, study in patients with HFpEF and HFmrEF, evaluating the effect of ziltivekimab 15mg compared with placebo, given once a month, in addition to standard care, on the primary composite outcome of time to first occurrence of cardiovascular death, heart .HERMES is a single-centre phase II trial of magnetic resonance-guided SBRT in men with localised prostate cancer . Forty-six men will be treated on the MR-linac and will be randomised between 36.25 Gy in five fractions over 10 days, with clinical target volume target of 40 Gy, and 24 Gy in two fractions over 8 days.
HERMES is an international collaboration to investigate the genetic basis of heart failure. This unique global effort currently comprises 57 population-based cohorts, case-control studies and randomized clinical trials, including over 140,000 heart failure cases. Recent randomised trials have demonstrated that anti-cytokine and anti-inflammatory therapies can reduce cardiovascular event rates, and evolving genetic, murine, translational, and human epidemiological data suggest that IL-6 . IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021 May 29;397 (10289):2060-2069. doi: 10.1016/S0140-6736 (21)00520-1. Epub 2021 May 17.
Die Studie beschreibt die angestrebte Lösung, in dem sie die groben Ziele definiert, möglichen Lösungsvarianten aufführt und diese dann bewertet. Sie bildet die Grundlage für die Entscheidung, ob ein Projekt freigegeben wird oder nicht.
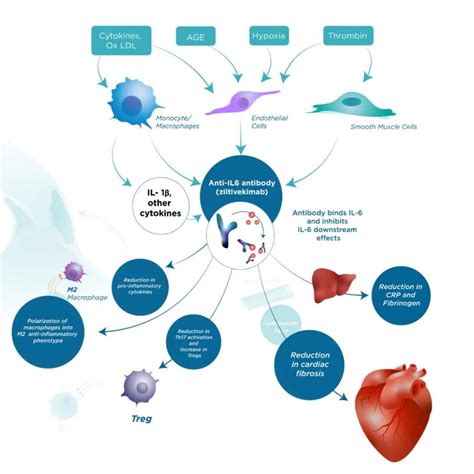
hermes (nct05636176) Effects of ziltivekimab versus placebo on morbidity and mortality in patients with heart failure with mildly reduced or preserved ejection fraction and systemic inflammation (HERMES).
In sum, based on the recent RESCUE data, the opportunity has arisen to move past CANTOS and IL-1β inhibition to address in ZEUS whether targeting IL-6 can provide even larger reductions in cardiovascular event rates.Background: We compared the tolerability and efficacy of erenumab, a monoclonal antibody binding to the calcitonin gene-related peptide receptor, to topiramate for migraine prophylaxis in adults. Methods: HER-MES was a 24-week, randomised, double-blind, double-dummy, controlled trial conducted in 82 sites in Germany.
Hermes cardiology
Launched in 1953, the Rolex Submariner is the first divers’ wristwatch to be waterproof to a depth of 100 metres. More on rolex.com.
hermes studie|ziltivekimab vs Hermes